Thalidomide
Thalidomide ATC Code L04AX02 : Antineoplastic and immunomodulating agents - Immunosuppressants drugs - Immunosuppressants drugs - Other immunosuppressants - Thalidomide
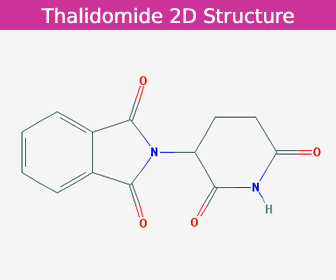
| Latin | Thalidomidum |
|---|---|
| Routes | Oral |
| Pregnancy category | X |
| FDA Approval Date | 1998-07-16 |
| EMA Approval Date | 2008-04-16 |
| Molecular Formula | C13H10N2O4 |
| Molecular Weight | 258.2295 g/mol |
| CAS Number | 50-35-1 |
Thalidomide list of brand/trade names
- Germany: Thalidomide Celgene
- Italy: Thalidomide Celgene
- Australia: Thalomid
- Belgium: Thalidomide Celgene
- Denmark: Thalidomide Celgene
- Greece: Thalidomide Celgene
- India: Oncothal, Thaloda and Thycad
- Ireland: Thalidomide
- Norway: Thalidomide Celgene
- Portugal: Thalidomide Celgene
- Sweden: Thalidomide Celgene
- Turkey: Thalidomide Er-Kim