Ticagrelor
Ticagrelor ATC Code B01AC24 : Blood and blood forming organs - Antithrombotic agents - Antithrombotic agents - Platelet aggregation inhibitors excluding heparin - Ticagrelor
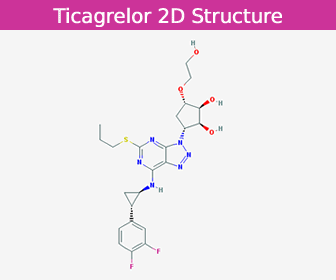
| Latin | Ticagrelorum |
|---|---|
| Routes | Oral |
| Pregnancy category | C |
| FDA Approval Date | 2011-07-20 |
| EMA Approval Date | 2010-12-03 |
| Molecular Formula | C23H28F2N6O4S |
| Molecular Weight | 522.568026 g/mol |
| CAS Number | 274693-27-5 |
Ticagrelor list of brand/trade names
- United States: Brilinta
- Germany: Brilique
- France: Brilique
- Spain: Brilique
- Italy: Brilique
- Canada: Brilinta
- Russia: Brilinta
- Australia: Brilinta
- Netherlands: Brilique and Possia
- Belgium: Brilique
- Denmark: Brilique
- Finland: Brilique
- Greece: Brilique
- Ireland: Brilique
- Norway: Brilique
- Portugal: Brilique
- Sweden: Brilique
- Switzerland: Brilique
- Indonesia: Brilinta
- Turkey: Brilinta